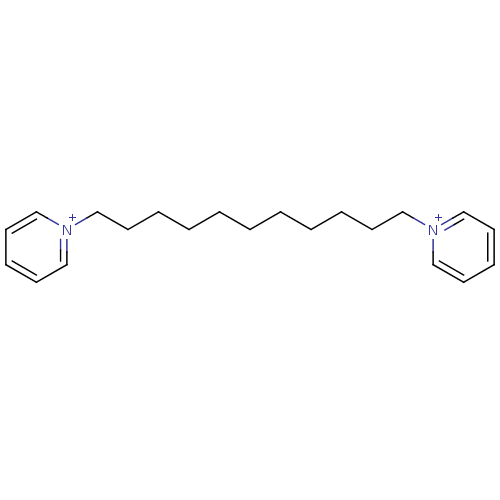
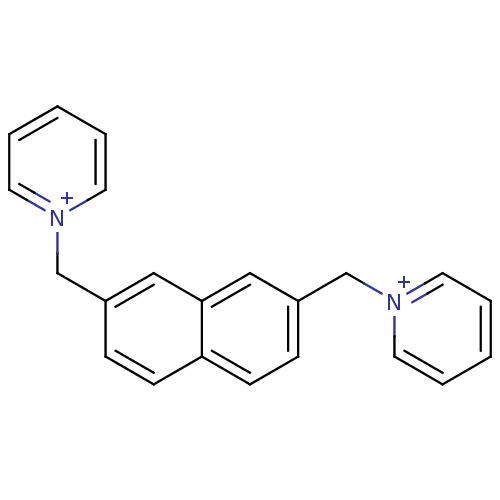
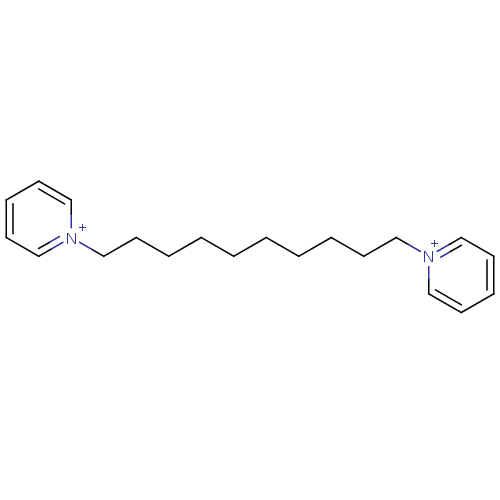
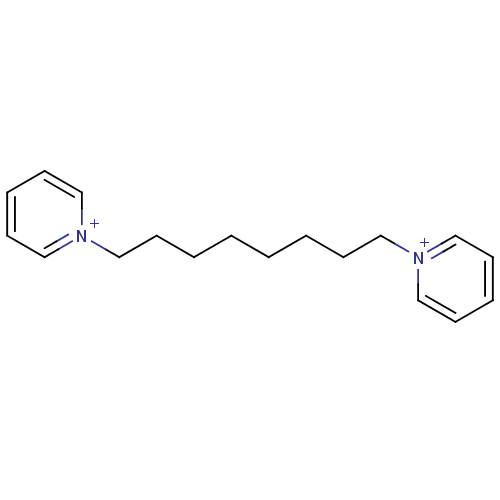
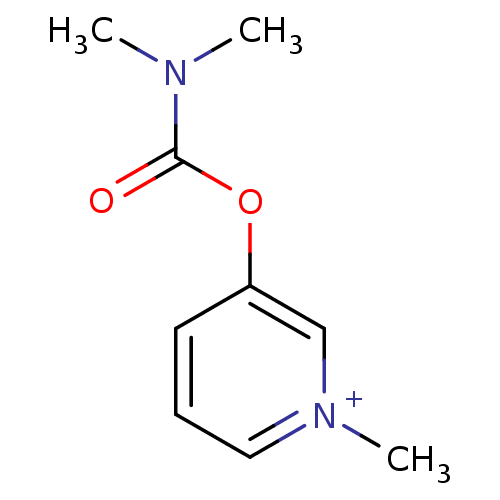
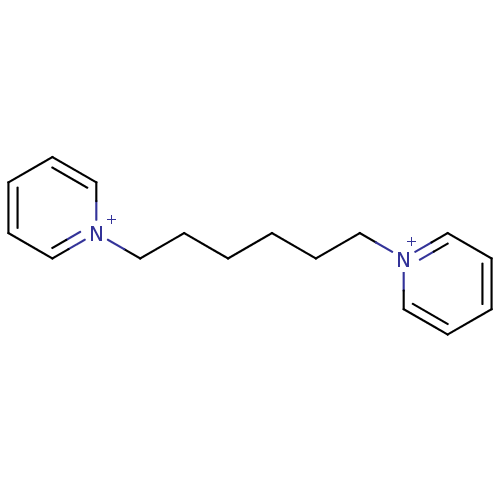
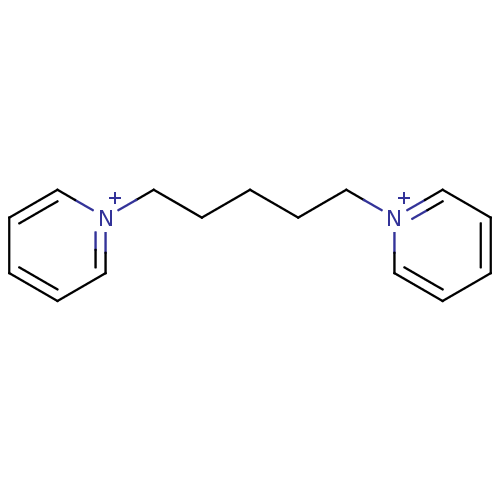
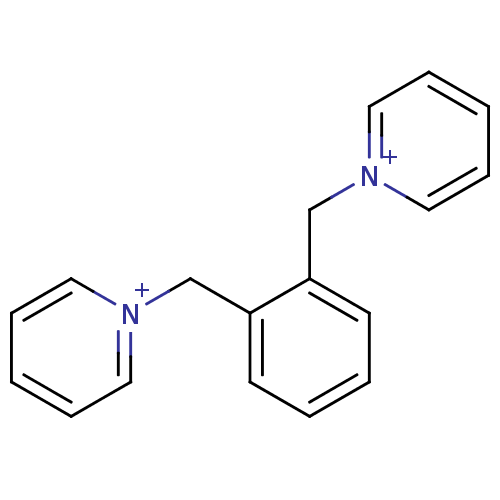
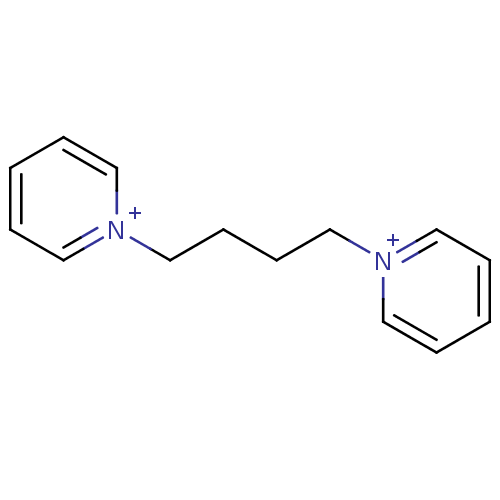
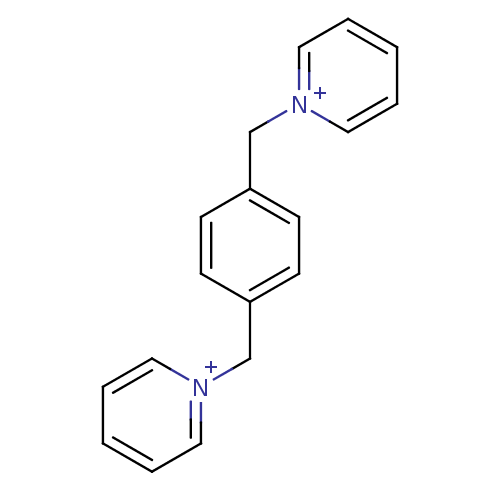
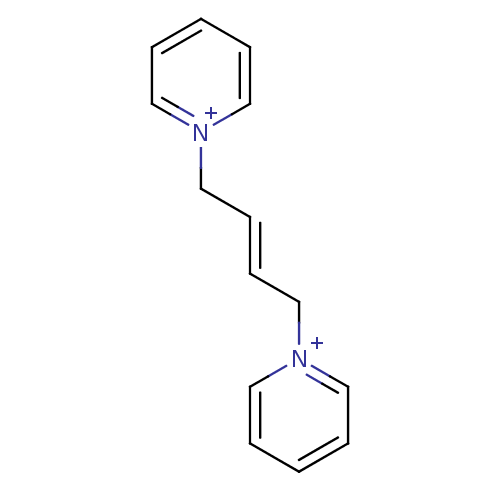
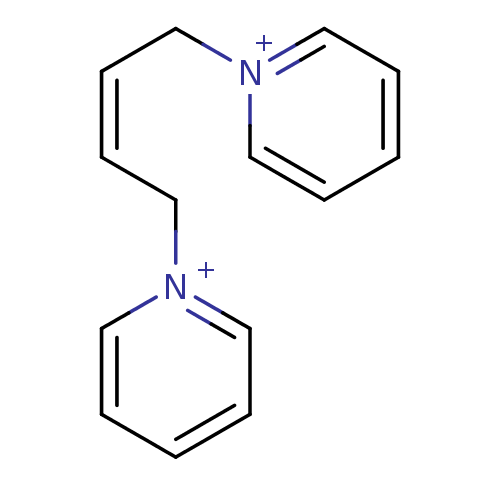
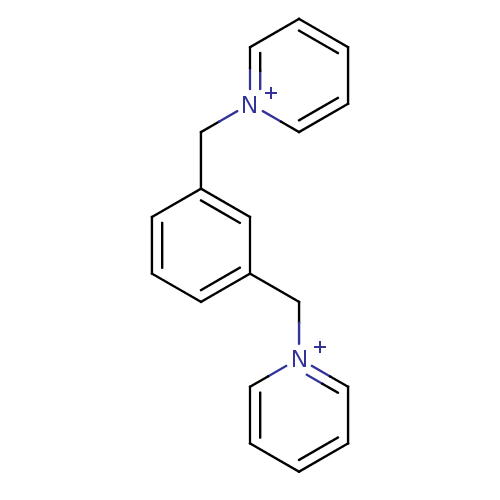
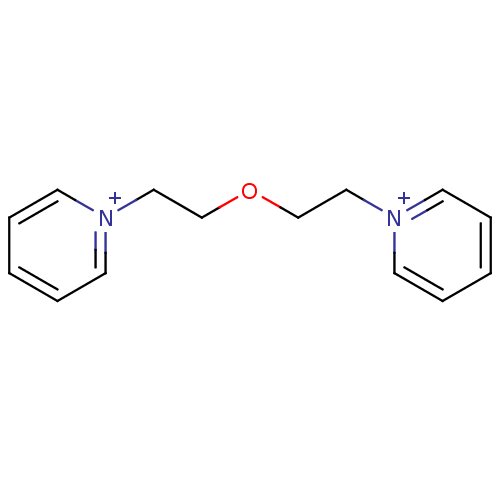
Affinity DataKi: 20nMAssay Description:Inhibition of human erythrocyte AChE after 5 mins by noncompetitive Lineweaver-burk plot analysis for enzyme-inhibitor complexMore data for this Ligand-Target Pair
Affinity DataKi: 30nMAssay Description:Inhibition of human erythrocyte AChE after 5 mins by noncompetitive Lineweaver-burk plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 140nMAssay Description:Inhibition of human erythrocyte AChE after 5 mins by noncompetitive Lineweaver-burk plot analysis for enzyme-inhibitor complexMore data for this Ligand-Target Pair
Affinity DataKi: 140nMAssay Description:Inhibition of human erythrocyte AChE after 5 mins by noncompetitive Lineweaver-burk plot analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Inhibition of human erythrocyte AChE after 5 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 200nMAssay Description:Inhibition of human erythrocyte AChE after 5 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 400nMAssay Description:Inhibition of human erythrocyte AChE after 5 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 700nMAssay Description:Inhibition of human erythrocyte AChE after 5 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 800nMAssay Description:Inhibition of human plasma BChE after 5 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 800nMAssay Description:Inhibition of human plasma BChE after 5 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of human erythrocyte AChE after 5 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+3nMAssay Description:Inhibition of human plasma BChE after 5 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 6.00E+3nMAssay Description:Inhibition of human plasma BChE after 5 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 7.00E+3nMAssay Description:Inhibition of human plasma BChE after 5 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.90E+4nMAssay Description:Inhibition of human plasma BChE after 5 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.10E+4nMAssay Description:Inhibition of human erythrocyte AChE after 5 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 4.00E+4nMAssay Description:Inhibition of human erythrocyte AChE after 5 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 6.30E+4nMAssay Description:Inhibition of human erythrocyte AChE after 5 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 7.80E+4nMAssay Description:Inhibition of human plasma BChE after 5 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+5nMAssay Description:Inhibition of human plasma BChE after 5 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.30E+5nMAssay Description:Inhibition of human plasma BChE after 5 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.41E+5nMAssay Description:Inhibition of human erythrocyte AChE after 5 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.45E+5nMAssay Description:Inhibition of human plasma BChE after 5 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 5.05E+5nMAssay Description:Inhibition of human erythrocyte AChE after 5 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 5.29E+5nMAssay Description:Inhibition of human plasma BChE after 5 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 5.41E+5nMAssay Description:Inhibition of human plasma BChE after 5 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 5.83E+5nMAssay Description:Inhibition of human plasma BChE after 5 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 6.36E+5nMAssay Description:Inhibition of human erythrocyte AChE after 5 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.27E+6nMAssay Description:Inhibition of human erythrocyte AChE after 5 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.27E+6nMAssay Description:Inhibition of human plasma BChE after 5 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.38E+6nMAssay Description:Inhibition of human plasma BChE after 5 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.54E+6nMAssay Description:Inhibition of human erythrocyte AChE after 5 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.77E+6nMAssay Description:Inhibition of human erythrocyte AChE after 5 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.99E+6nMAssay Description:Inhibition of human erythrocyte AChE after 5 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.01E+6nMAssay Description:Inhibition of human erythrocyte AChE after 5 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.29E+6nMAssay Description:Inhibition of human erythrocyte AChE after 5 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.36E+6nMAssay Description:Inhibition of human erythrocyte AChE after 5 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.36E+6nMAssay Description:Inhibition of human plasma BChE after 5 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 9.80E+6nMAssay Description:Inhibition of human plasma BChE after 5 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.60E+7nMAssay Description:Inhibition of human plasma BChE after 5 mins by Ellman's methodMore data for this Ligand-Target Pair